Corrosion is a significant issue that affects everything from vehicles to industrial machinery, leading to the deterioration of valuable assets. While rust is commonly seen as a problem, the causes and solutions are less understood. However, knowing the science behind corrosion opens the door to effective solutions like Cortec’s Vapor phase Corrosion Inhibitors (VpCIÂź), which help preserve metal surfaces in industrial applications.
What Is Corrosion?
Corrosion is a natural electrochemical process where metals return to their original, oxidized state, such as rust on steel. Steel, for example, is derived from iron ore, which contains iron oxidesâits natural form of rust. Producing steel requires significant energy to convert iron ore into the valuable material. Over time, however, the steel inevitably reverts to iron oxide due to natural forces, resulting in corrosion.
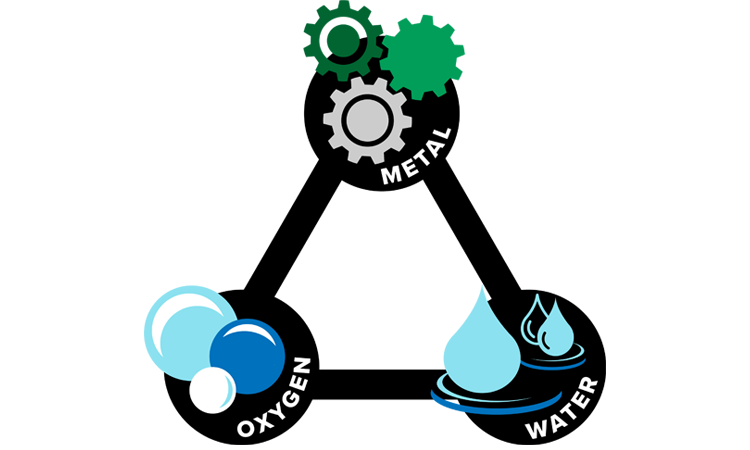
The Corrosion Triangle and How to Stop It
The key to preventing rust lies in understanding the corrosion triangle. For corrosion to occur, three components are needed: metal, oxygen, and an electrolyte. When oxygen comes into contact with metal in the presence of water or an electrolyte, it triggers an electrochemical reaction that causes rust. By breaking the connection between any of these three elements, corrosion can be halted. Without oxygen, there can be no oxidation; without an electrolyte, the electrochemical process can’t complete; and without metal, there’s nothing to corrode.
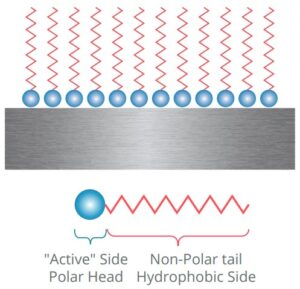
How VpCIÂź Interrupts the Corrosion Process
Cortec’s Vapor phase Corrosion Inhibitors (VpCIÂź) disrupt the corrosion triangle by creating a protective molecular barrier that prevents the interaction of metal with moisture and oxygen. These inhibitors, such as amine carboxylates, have an affinity for metal surfaces and can “stick” to them, forming a thin protective layer. This layer effectively blocks oxygen and moisture from interacting with metal ions, much like filling up all the seats at a table, preventing corrosion-causing reactions from occurring.
The Vapor-Phase Application Mechanism
One of the unique advantages of VpCIÂź is its vapor-phase application. These inhibitors can sublimate from liquid or solid form into a vapor, spreading throughout the enclosed space to reach areas difficult to access. Unlike traditional greasy rust preventatives, VpCIÂź vapors do not require removal after application and can be applied easily to complex or intricate surfaces.
For example, metal components can be wrapped in VpCIÂź-coated paper or placed inside a VpCIÂź-126 Blue bag, which protects them from rust. In electrical applications, VpCIÂź-111 Emitters can be placed inside enclosures, where vapors circulate, protecting sensitive metal parts like wires and contacts. Additionally, waterborne VpCIÂź can be fogged into large equipment such as heat recovery steam generators during layup to prevent rust without disrupting future use.
A Powerful Solution for Rust Prevention
Recognizing rust is easy, but understanding how to prevent it is key to protecting valuable assets. VpCIÂź offers one of the most efficient and hassle-free methods to combat corrosion in industrial environments. By breaking the corrosion cycle and creating a barrier between metal and harmful elements, VpCIÂź technology provides robust and long-lasting protection for metals in enclosed spaces.
To learn more about how Cortecâs Vapor phase Corrosion Inhibitors can safeguard your equipment, visit Cortec’s website.
CortecÂź Corporation is a global leader in corrosion control technologies, offering a wide range of VpCIÂź and MCIÂź solutions for industries such as packaging, metalworking, construction, electronics, and more. Committed to sustainability, quality, and service, Cortec manufactures over 400 products and serves customers worldwide from its headquarters in St. Paul, Minnesota. Cortec is ISO 9001:2015, ISO 14001:2015, and ISO/IEC 17025:2017 certified.
Contact:Â CortecÂź Corporation
Website:Â www.cortecvci.com